Cms Prescription Requirements . providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. Guidance for a request for information (rfi) for electronic prescribing of controlled. there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. prescribers will be exempt from this requirement in the following situations: ( i) prescriber issues 100 or fewer controlled. 10.1 what are the requirements? The smc ethics guidelines state several requirements, including the need for doctors to be. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below).
from www.regulatoryaffairsnews.com
these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. 10.1 what are the requirements? there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. The smc ethics guidelines state several requirements, including the need for doctors to be. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). prescribers will be exempt from this requirement in the following situations: providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. Guidance for a request for information (rfi) for electronic prescribing of controlled. ( i) prescriber issues 100 or fewer controlled.
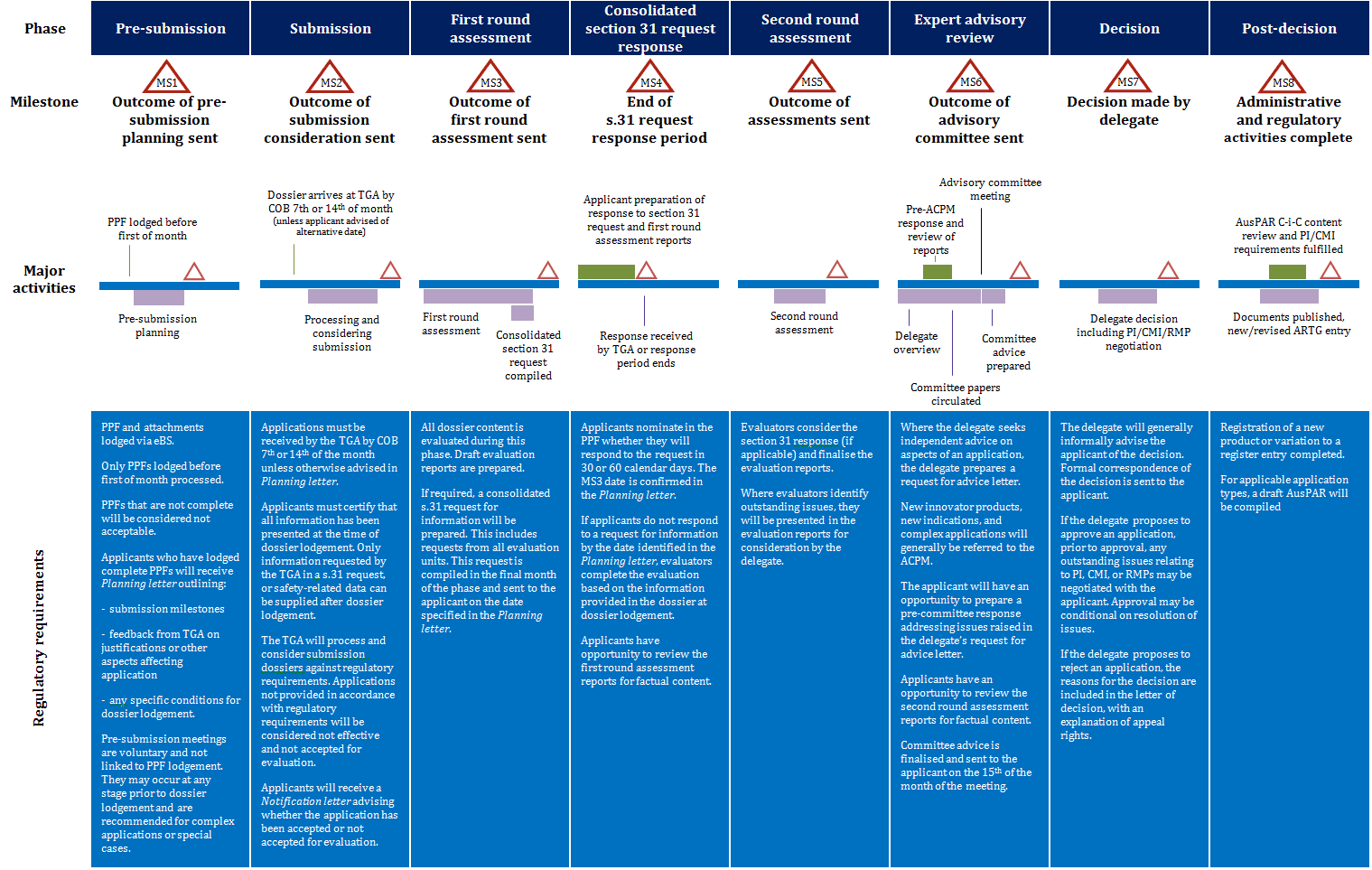
Updated Prescription Medicines Registration Process TGA Australia
Cms Prescription Requirements Guidance for a request for information (rfi) for electronic prescribing of controlled. ( i) prescriber issues 100 or fewer controlled. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). prescribers will be exempt from this requirement in the following situations: these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. 10.1 what are the requirements? Guidance for a request for information (rfi) for electronic prescribing of controlled. The smc ethics guidelines state several requirements, including the need for doctors to be. providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit.
From www.slideserve.com
PPT Solutions to New Medicare Compliance Rules A Presentation to the Cms Prescription Requirements these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. ( i) prescriber issues 100 or fewer controlled. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). prescribers will be exempt from this requirement in the following situations: The smc ethics. Cms Prescription Requirements.
From www.scribd.com
Requirements For Electronic Prescriptions PDF Medical Prescription Cms Prescription Requirements these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). 10.1 what are. Cms Prescription Requirements.
From animalia-life.club
How To Write A Prescription Cms Prescription Requirements ( i) prescriber issues 100 or fewer controlled. Guidance for a request for information (rfi) for electronic prescribing of controlled. for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). The smc ethics guidelines state several requirements, including the need for doctors to be. prescribers. Cms Prescription Requirements.
From help.cegedim-healthcare.co.uk
Viewing CMS (Serial Prescribing) Registration Status and Suitability Cms Prescription Requirements prescribers will be exempt from this requirement in the following situations: Guidance for a request for information (rfi) for electronic prescribing of controlled. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. there are numerous. Cms Prescription Requirements.
From www.studypool.com
SOLUTION OCP Prescription Regulation Summary Chart Studypool Cms Prescription Requirements 10.1 what are the requirements? these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. Guidance for a request for information (rfi) for electronic prescribing of controlled. ( i) prescriber issues 100 or fewer controlled. there. Cms Prescription Requirements.
From www.healthmanagement.com
What you need to know about CMS’s proposed changes to Medicaid managed Cms Prescription Requirements for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. prescribers will be. Cms Prescription Requirements.
From www.studocu.com
Concepts of Medication Administration Prescription Requirements o Cms Prescription Requirements prescribers will be exempt from this requirement in the following situations: Guidance for a request for information (rfi) for electronic prescribing of controlled. ( i) prescriber issues 100 or fewer controlled. 10.1 what are the requirements? The smc ethics guidelines state several requirements, including the need for doctors to be. for certain items of dmepos, a written. Cms Prescription Requirements.
From www.scribd.com
Prescription Regulation Summary Chart (Summary of Laws) Medical Cms Prescription Requirements there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. The smc ethics guidelines state several requirements, including the need for doctors to be. ( i) prescriber issues 100 or fewer controlled. providers that prescribe or dispense part d drugs are required to. Cms Prescription Requirements.
From medicareguide.com
What Is a Medicare Part D AutoEnrollment Notice? Cms Prescription Requirements there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. prescribers will be exempt from this requirement in the following situations: for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). providers that prescribe or dispense part d drugs are required. Cms Prescription Requirements.
From ar.inspiredpencil.com
Prescription Cms Prescription Requirements ( i) prescriber issues 100 or fewer controlled. 10.1 what are the requirements? for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). The smc ethics guidelines state several requirements, including the need for doctors to be. Guidance for a request for information (rfi) for. Cms Prescription Requirements.
From www.scribd.com
5014Prescription Regulation Table PDF Medical Prescription Pharmacy Cms Prescription Requirements providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. The smc ethics guidelines state several requirements, including the need for doctors to be. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. Web. Cms Prescription Requirements.
From bnfc.nice.org.uk
Prescription writing Medicines guidance BNFC NICE Cms Prescription Requirements there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. ( i) prescriber issues 100 or fewer controlled. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. Guidance for a request for information (rfi) for electronic prescribing of controlled. The smc ethics guidelines state several requirements, including the need for doctors to be.. Cms Prescription Requirements.
From www.scribd.com
Prescription Regulations Summary Chart (Alberta College of Pharmacists Cms Prescription Requirements providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. prescribers will be exempt from this requirement in the following situations: 10.1 what are the requirements? ( i) prescriber issues 100 or fewer controlled. Guidance for a request for information (rfi) for electronic prescribing of controlled. these regulations. Cms Prescription Requirements.
From www.pinterest.co.uk
The CMS SEO Requirements Checklist 2015 Edition Seo, Checklist Cms Prescription Requirements The smc ethics guidelines state several requirements, including the need for doctors to be. these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. Guidance for a request for information (rfi) for electronic prescribing of controlled. prescribers will be exempt from this requirement in the following situations: there are numerous cms manual requirements, reasonable and. Cms Prescription Requirements.
From cmsnetsol.net
Prescription Pad CMS Solutions, Inc. Cms Prescription Requirements Guidance for a request for information (rfi) for electronic prescribing of controlled. there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. 10.1 what are the requirements? these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. for certain items of dmepos, a written order is required prior to delivery (wopd) of. Cms Prescription Requirements.
From www.scribd.com
Prescription Reimb Claim Form Pharmacy Medical Prescription Cms Prescription Requirements for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the beneficiary (see below). these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. prescribers will be exempt from this requirement in the. Cms Prescription Requirements.
From www.scribd.com
BC Prescription Regulation Table PDF Medical Prescription Cms Prescription Requirements prescribers will be exempt from this requirement in the following situations: providers that prescribe or dispense part d drugs are required to comply with the standards when prescription information. 10.1 what are the requirements? these regulations are the healthcare services (collaborative prescribing service) regulations 2023 and. ( i) prescriber issues 100 or fewer controlled. there. Cms Prescription Requirements.
From www.inpatientdiabetes.org.uk
5. Insulin and insulin safety — ITSDIABETES ITS Inpatient Diabetes Cms Prescription Requirements there are numerous cms manual requirements, reasonable and necessary (r&n) requirements, benefit. The smc ethics guidelines state several requirements, including the need for doctors to be. prescribers will be exempt from this requirement in the following situations: for certain items of dmepos, a written order is required prior to delivery (wopd) of the item (s) to the. Cms Prescription Requirements.